Wajahat Abbas/Published - June 2025
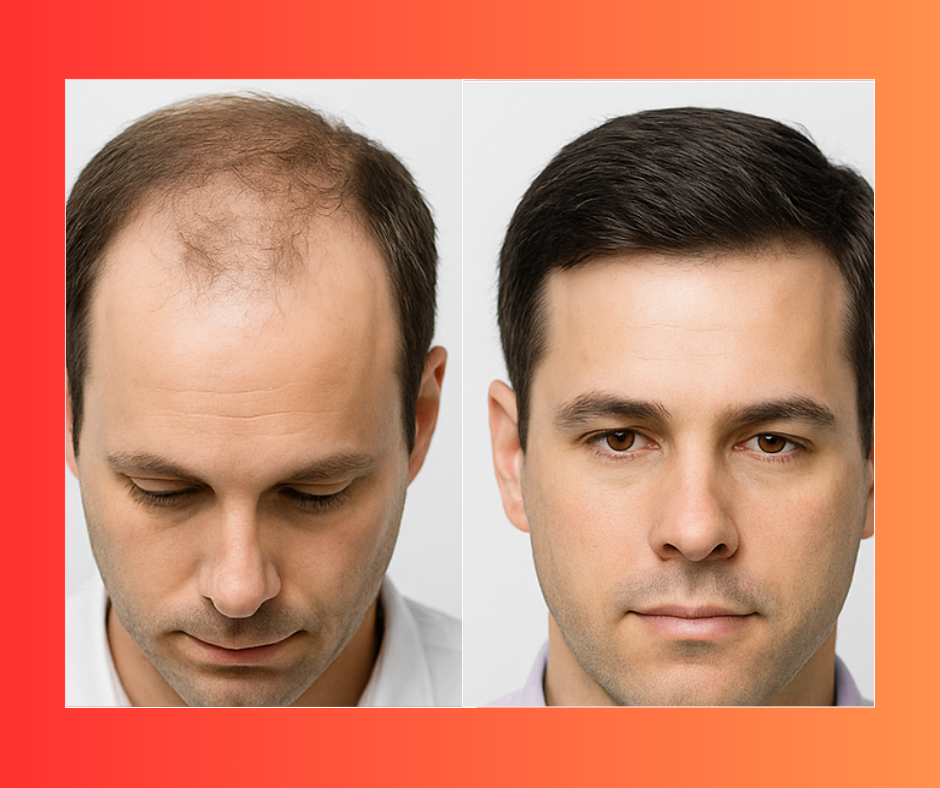
The potential? A safe, non-invasive way to regrow your own hair, making baldness reversible for millions of people. The Lab tests showed mice completely regrew hair in just 10 days using a simple topical treatment. No pills. No implants. No surgery. Early human trials are already underway in 2025, and the results are turning heads in the scientific world. Could this be the future of hair restoration? All signs say yes.
The potential? A safe, non-invasive way to regrow your own hair, making baldness reversible for millions of people. The Lab tests showed mice completely regrew hair in just 10 days using a simple topical treatment. No pills. No implants. No surgery. Early human trials are already underway in 2025, and the results are turning heads in the scientific world. Could this be the future of hair restoration? All signs say yes.
Why This research Is a Game-Changer
- No surgery or injections required
- No hormonal interference or genetic alteration
- Visible results in as little as 10 days (in mice)
- Clinically tested and well tolerated in humans
- Backed by UCLA and supported by Google Ventures
What Is PP405 Molecule, and How Does it Work?
Hair follicles contain stem cells crucial for hair growth. These stem cells become inactive due to aging, stress, or hormonal changes. PP405 activates the same SCUBE3 signaling pathway that tells stem cells to “wake up. As a result: Dormant hair follicles regenerate and begin producing healthy hair again. “We didn’t just see a few strands come back. We saw dense, consistent growth across treated areas,” said UCLA lead researcher Dr. William Lowry
Clinical Trials & Research Summary
1. Phase 2a Clinical Trial (2025)
In February 2025, Pelage Pharmaceuticals—a UCLA spin-off—launched Phase 2a trials to test PP405 in individuals with androgenetic alopecia (pattern baldness). Around 60 participants of various hair types and skin tones were enrolled. Each applied the topical gel daily for 16 weeks in a randomized, double-blind, placebo-controlled study.
2. Phase 1 Human Trial (2023)
In 2023, UCLA conducted Phase 1 trials in Orange County, California, where participants applied a low-concentration topical PP405 gel each night for one week. The treatment was well tolerated with no serious side effects, and importantly, it reactivated dormant follicles without damaging existing ones. Lab analysis showed increased stem cell activity and follicle regeneration.
Dr. Lowry’s Insights on Human Trial Results
Dr. William Lowry, a UCLA professor and scientific co-founder of Pelage Pharmaceuticals, described the initial human data on PP405 as “very encouraging”—emphasizing that the compound not only reactivated dormant hair follicles, but did so safely without harming existing ones. Reflecting on early concerns, he admitted
Clinical Trial Details & Funding
The PP405 trials are led by Pelage Pharmaceuticals, a UCLA spin-off. Following positive Phase 1 results, Google Ventures (GV) invested $14 million to fund Phase 2a development in 2025. The ongoing trial, registered as NCT06393452, is designed to evaluate safety and efficacy across a diverse group of participants.
Phase 1 Trial (2023): Conducted in Orange County, CA, with topical application of 0.05% PP405 gel. Results showed stem cell activation without systemic absorption.
Phase 2a Trial (NCT06393452): Initiated in February 2025 with approximately 60 participants over a 16-week study. Registered at ClinicalTrials.gov.**: Final results anticipated by the end of 2025.